 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
Research Interests: Inorganic Reactions in Ionic Liquids
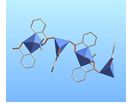
The vast majority of inorganic syntheses are done in either aqueous or organic solution. The objective of this study is to perform inorganic reactions in very different types of solvent system, ionic liquids. Ionic liquids are different from other solvents in that they are composed of charged particles, much like common salts such as NaCl. These salts differ from common salts in that they have an unusually low melting point. Most common salts have melting points of the order of several hundred deg C. Ionic liquids, as implied by the name, have melting points below room temperature; however, their boiling points are of the order of several hundred degrees due to their ionic nature. At room temperature the ionic liquid consists of freely moving ions creating a very different environment for chemical reactions. These ionic liquids prove to be excellent solvents for chemical reactions for a number of reasons but mostly because they dissolve a wide range of inorganic and organic substances, so unusual combinations of reagents can be brought together in the same phase. Another interesting property that we’ve discovered in our research is that ionic liquids facilitate the crystallization of many inorganic compounds. In inorganic chemistry, crystallization is often a necessary and difficult step of a chemical synthesis. The only definitive way to fully characterize many inorganic compounds is to get an X-ray crystal structure (which means that you first need to grow crystals!). Because compounds are frequently more soluble in ionic liquids, products precipitate very slowly, leading to larger, more well-formed crystals. On several occasions, we have encountered syntheses that involve a complicated recrystallization process. However, when we performed these reactions in ionic liquids, crystals grow well just by letting the solution sit for a period of time.
Our
overall goal is to synthesize inorganic complexes containing multiple
metal centers, specifically those containing both a lanthanide and a
transition metal. Metal complexes with large numbers of metal
centers are interesting to the scientific community for a number of
reasons: (1) because they represent new and interesting structural types
(2) because they are precursors to high temperature superconductors,
and (3) because they contain unpaired electrons that may interact with
one another and lead to unusual magnetic properties. Syntheses
of examples of such multinuclear metal complexes are well-documented
in the chemical literature
We attempt
to synthesize new and interesting complexes containing transition
metals, lanthanides, and squarate, pyridonate, and/or picolinate
ligands in ionic liquids. These ligands are particularly interesting
because they have many possible binding modes so many different structural
types are possible. Such ligands can bridge, chelate, or bridge
and chelate. We first have to synthesize our own ionic liquids,
such as ethyl ammonium nitrate
(some we actually purchase). We will
try a variety of permutations of the synthesis reactions, changing such
variables as (1) order of addition of reagents, (2) ratios of the transition
metal to lanthanide to ligand, (3) choice of ionic liquid (or other
solvent), (4) choice of auxiliary ligands. We have already seen
the dramatic effects of changing these variables this semester.
Reactions that yield solid products will be characterized by NMR and
IR spectroscopy. If this characterization implies a potential new and
interesting compound, the crystals can be sent for elemental analysis.
The final step in the characterization process will be to send the crystals
for X-ray crystal structure determination. If the results indicate
a novel complex, further magnetic susceptibility and electrochemistry
characterization will be performed. Due to the lack of these particular
types of complexes in published literature, almost any original compounds
formed during our research will be excellent candidates for publication
in a chemical journal.
My students frequently present their research at local and national American Chemical Society Meetings in places like Indianapolis, Atlanta, and San Francisco.
| DePauw University | ||||