Chemistry of Hemoglobin:
Heme has a central Fe2+ supported by four porphyrin N atoms. Each of the four globin subunit also codes for a histidine, which contains a five-membered imidazole ring with a nitrogen-donor atom. This donor-nitrogen bonds to Fe2+ of heme, thus cooardinating the heme from a side. The vacant side of the is where O2 binds as a ligand, forming an octahedron.
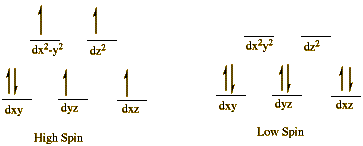
In the , the Fe2+ has an electronic configuration of t2g4eg2, which makes it a high-spin. In this state, Fe2+ has a substantially larger radius than in the low-spin state. The increased radius makes it too big to fit in the porphyrin-ligand hole; thus, it sits above the plane of porphyrin ring.
When to the heme, it adds just enough strength to the ligand field to convert the Fe2+ from high-spin state to low-spin state. This bonding of O2 causes Fe2+ to decrease its radius by 17pm, which makes it small enough to fit in the porphyrin ring. Since the protein is attached to the iron through the histidine, the transfer of iron into the porphyrin hole pulls the globin to change its conformation. This motion is eventually passed to the other three globins, sending a message that an O2 has been attached and they should do likewise. This process of transmitting message within the subunits is known as cooperativity.