|
Links |
Molecular/Biochemical Properties and Characteristics of Cri Du Chat-Related Proteins | |
|
Initial Discovery and Clinical Aspects Molecular/Biochemical Properties and Characteristics of Cri Du Chat-Related Proteins Links to Cri Du Chat Support Groups
|
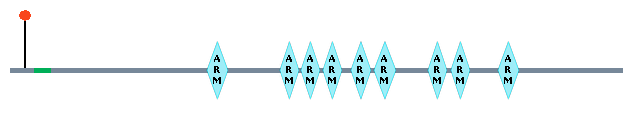
Molecular/Biochemical properties and characteristics of the protein To date, three genes that code for proteins are suspected to be or are directly involved in the phenotypes of CdCS. These proteins, delta-catenin, Semaphorine F, and telomere reverse transcriptase, which participate in important biological processes of the body, can cause phenotypic symptoms of CdCS due to deletion of gene sequence coding for these proteins. These will be investigated more thoroughly below. Delta-catenin Delta-catenin, which is a product of the gene CTNND2 at 5p15.2, is an adherence junction protein involved in cell motility and expressed early in neuronal development. This protein is composed to 1225 amino acids which contain repeats called “Armadillo” repeats within this sequence. These repeats are usually 42-45 amino acids in length. The domain of this protein consists of a multi-helical fold comprised of two curved layers of alpha helices arranged in a regular right-handed superhelix, where the repeats that make up this structure are arranged about a common axis. These super-helical structures present an extensive solvent-accessible surface that is well suited to binding large substrates such as proteins and nucleic acids.
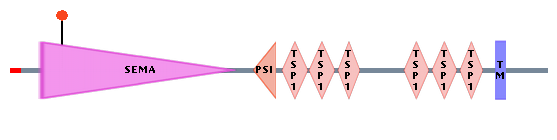
A deletion of this chromosomal region has been associated with the cri-du-chat syndrome. delta-catenin maps to a specific region in 5p15.2 that has been implicated in the mental retardation phenotype of CdCS. The breakpoints in patients with 5p terminal deletions were characterized with respect to the severity of mental retardation and the physical location of the delta-catenin gene. A strong correlation was found between the hemizygous loss of delta-catenin and severe mental retardation. These findings and the properties of delta-catenin as a neuronal-specific protein, expressed early in development and involved in cell motility, support its role in the mental retardation of CdCS when present in only one copy. One can find the OMIM DNA sequence for delta-catenin here. Semaphorine F Semaphorine F, coded from the SEMAF gene, is a protein involved in axonal guidance during neural development. This 1,077-amino acid protein contains an N-terminal signal sequence, followed by a semaphorin domain, 7 thrombospondin type-1 repeats a putative transmembrane domain, and a short cytoplasmic tail. It also has 13 conserved cysteines and a conserved N-glycosylation site.
The semaphorin domain belongs to a large family of secreted and transmembrane proteins, some of which function as repellent signals during axon guidance. Sema domains also occur in a hepatocyte growth factor receptor, in SEX protein and in viral proteins. Simmons et al. (1998) used a YAC gene library of the human genome to map the SEMAF gene to chromosome 5p15.2. They determined that the SEMAF gene covers at least 10% of the CdCS critical region, and therefore, its deletion can possible be responsible for phenotypes of CdCS patients. Telomere Reverse Transcriptase Telomere Reverse Transcriptase is a ribonucleoprotein polymerase that maintains telomere ends by addition of the telomere repeat TTAGGG. The enzyme consists of a protein component with reverse transcriptase activity, encoded by this gene, and an RNA component, which serves as a template for the telomere repeat. Telomerase expression plays a role in cellular senescence, as it is normally repressed in postnatal somatic cells resulting in progressive shortening of telomeres.
(taken from http://www.bio.davidson.edu/Courses/Molbio/MolStudents/spring2003/Parker/protein.html) Cri du chat syndrome (CdCS) results from loss of the distal portion of chromosome 5p, where the telomerase reverse transcriptase (hTERT) gene is localized (5p15.33). hTERT is the rate-limiting component for telomerase activity that is essential for telomere-length maintenance and sustained cell proliferation. Here, we show that a concomitant deletion of the hTERT allele occurs in all 10 patients with CdCS whom we examined. Induction of hTERT mRNA in proliferating lymphocytes derived from five of seven patients was lower than that in unaffected control individuals. Reconstitution of telomerase activity by ectopic expression of hTERT extended the telomere length, increased the population doublings, and prevented the end-to-end fusion of chromosomes. We conclude that hTERT is limiting and haploinsufficient for telomere maintenance in humans in vivo. Accordingly, the hTERT deletion may be one genetic element contributing to the phenotypic changes in CdCS. Telomere shortening is one postulated basis for replicative senescence, via down-regulation of telomerase reverse transcriptase (TERT); telomere dysfunction also is associated with greater sensitivity to apoptosis. Forced expression of TERT in cardiac muscle in mice was sufficient to rescue telomerase activity and telomere length. The deletion of the sequence of hTERT gene can also be responsible for cardiac defects phenotypes (eg, ventricular septal defect [VSD], atrial septal defect [ASD], patent ductus arteriosus [PDA], tetralogy of Fallot) of CdCS.
|